1.1. Structure of the Physical World¶
Physics is no less than the study of what everything around us is made out of and how it works, from the tiniest pieces of the microscopic world to the vast stretches of the cosmos. The scope and ambition of such a study is so enormous that it can be bewildering to know where to start. Indeed, the historical path towards our current understanding has been long and winding, with many fascinating twists and dead-ends.
A particularly productive strategy for achieving physical understanding has been to systematically break down the seemingly complicated and ineffable world we see around us into its most basic pieces. The idea is that after understanding the simplest pieces, more complicated arrangements of those pieces can then be tackled. Such a “divide and conquer” strategy for grounding our understanding follows a philosophy known as reductionism.
A reductionistic endeavor is akin to taking apart a watch or other piece of technology to identify and categorize all its most basic parts, then reassembling it again to gain insight into how those parts work together to create the whole. We don’t achieve a full understanding of the complicated whole from its pieces alone, but we gain rich insight into the basic mechanisms for how the pieces must fit together to produce that whole. Reductionism has been an incredibly pragmatic and productive perspective for the study of physics. It provides a firm foundation for building nuanced understanding of larger and more complicated physical scenarios.
1.1.1. The Standard Model¶
Over the past several hundred years, we have managed to reduce all that we see around us to a shockingly small number of “fundamental” things. They are fundamental in the sense that they do not appear to have any further substructure, at least that we have been able to observe so far. These fundamental pieces form The Standard Model of our physical world and describe not only the substance we see around us (called matter) but also the ways those substances can change (called interactions).
The Standard Model has been verified in high-energy physics experiments using colliders that quite literally smash objects together at faster and faster speeds to see what smaller pieces fly out of the collision to impact detectors placed around the collision location. The most stringent recent tests have been performed at the Large Hadron Collider (LHC). Remarkably, there have been almost no significant discrepancies between the predictions of the Standard Model and the unprecedented amount of data collected at the LHC since 2008 (over 330 million Gigabytes so far). Indeed, most theoretical proposals for going beyond the Standard Model have either been ruled out entirely or made much less plausible by the experimental data. The Standard Model is thus the most well-tested and well-verified reductionist physical model in human history.
Everything we see around us, including the material objects we bump into, the water we drink, the air we breathe, and the sunlight we bask in, can be reduced to the fundamental pieces of the Standard Model. All the chemical elements in the Periodic Table are formed from more basic pieces in the Standard Model. All the forces, electrical power, nuclear radiation, internet data streams, and other physical phenomena around us arise from the behavior of the pieces in the Standard Model.
Despite the vast scope of its explanatory power, the Standard Model is concise enough to be summarized in a small diagram:
While we will unpack this table in more detail later, but the following video provides a fun initial tour of the basic components of the Standard Model for now:1.1.2. A Brief History of Physical Discoveries¶
To help give some context behind the construction of this remarkably compact description of the world, let us review a brief and selective history of scientific discoveries in physics over the past century:
1.1.2.1. Elements¶
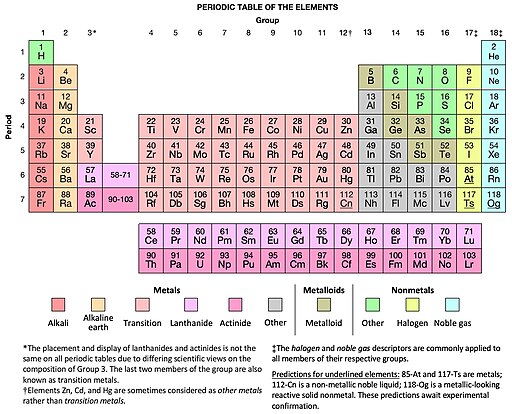
By around 1900CE chemists and physicists had reduced the complexity of all material substance (or matter) that we see to a set of basic elements, like gold, silver, copper, iron, carbon, and hydrogen. Each of these elements had been identified and refined throughout history in more and more pure forms. Their reactions and interactions had been studied extensively by alchemists and chemists, while their mechanical and thermodynamical properties had been investigated by physicists.
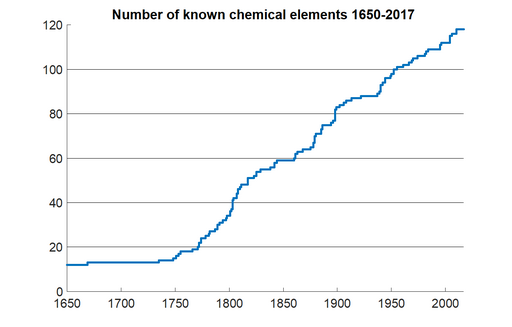
In 1869, the chemist Mendeleev began arranging the 64 elements known at that time into a table based on periodicities he observed in how the various elements reacted chemically. This initial table, and similar concurrent efforts, were then successively refined by Wilson and other chemists such that by 1905 a 32-column form of the (then 84) basic elements had reached a relatively stable form. This table is now known as the Periodic Table of the Elements, which has now merged our best physical and chemical understandings of the elements into one comprehensive and informative display.
As of 2019, we have verified 118 distinct elements. The 92 elements up through Uranium occur naturally, while the remaining “transuranic” elements are unstable and thus exist only temporarily in laboratory settings before decaying to other elements.
Though it was initially unclear why, the periodic table approximately splits naturally into a set of “blocks” with distinct chemical behavior. These blocks are now traditionally labeled “s”, “p”, “d”, and “f” to match prior labels of their spectroscopic emission lines as “sharp”, “principle”, “diffuse”, and “fundamental.”
1.1.2.2. Atoms, Molecules, Solids, Liquids, and Gases¶
The origins of the elements were unknown, so they were (erroneously) treated as fundamental. However, it was observed that each element had a “smallest piece” that could be isolated, such that larger chunks of that element were composed of many such pieces. Following Greek philosophy, these indivisible pieces of the elements were called atoms.
Small collections of atoms being held together by (electromagnetic) bonds are called molecules. Larger collections of atoms and molecules form various phases/states of matter, the most common of which are gases, liquids, and solids. The macroscopic distinctions between the states of matter correspond to microscopic features of the atoms and molecules, such as how close the molecules are to each other (pressure) and how rapidly they are moving/vibrating (temperature). Changing these microscopic features produces phase transitions between the different states of matter.

The molecules in solid crystals are close together and jiggling slowly, so form tightly ordered lattice structures. Such crystals tend to be periodic with particular lengths along distinct directions, or quasi-periodic with multiple nested periodicities of different atomic types interspersed.
For example, the following picture (from a scanning transmission electron microscope) shows interspersed cubic lattices of Strontium atoms (lighter color) and Titanium atoms (darker color):
Molecules in liquids are more loosely bound so form disordered and non-periodic arrangements that can flow and deform to fill the shapes of containers. Puzzlingly, molecules in glasses are similarly disordered but more tightly bound with widely varying and quasi-solid properties that are difficult to predict. Understanding glasses better is still an active research topic in “condensed matter physics” and “materials science.”

The molecules in gases are rapidly moving and diffuse so fly around individually to bounce off each other at high speeds, thus filling and bouncing off all interior surfaces of closed containers.
The way that a state of matter changes when qualities like pressure and temperature are varied is quite complicated, as illustrated in the following phase diagram for the behavior of water. Though simple transitions like melting, freezing, evaporation, and condensation are well-known in daily life near standard atmospheric pressure, more exotic things can happen at higher and lower pressures.
More exotic phases of matter like plasmas, superfluids, and condensates require a more detailed understanding of atomic structure that goes beyond simple atomic and molecular structure and motion.
1.1.2.3. Light as Electromagnetic Waves¶
Concurrently with the development of the Periodic Table, physicists had been developing the theories of electricity, magnetism, and optics during the 1800’s. Shockingly, all three investigations were unified into a single theory of electromagnetism by Maxwell in 1862, which was fully verified experimentally by 1890. Rather than being distinct physical effects, electricity, magnetism, and light were all manifestations of the same fundamental thing. Notably, Maxwell established that light was an electromagnetic wave that was self-generating as it propagated through space at a fixed speed \(c\) (which will become an important speed later).
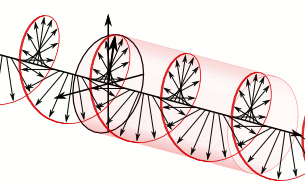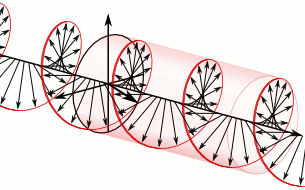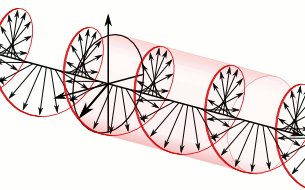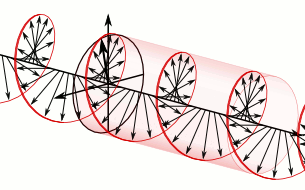
We now understand that the natural modes of electromagnetic wave propagation are helical (or circularly polarized). In such a wave, both electric and magnetic parts orbit around an axis of propagation. These directional parts of the wave push any electric charges and currents they encounter.
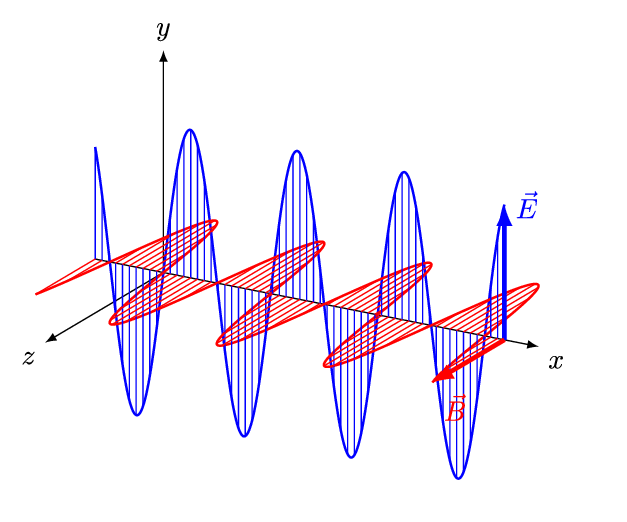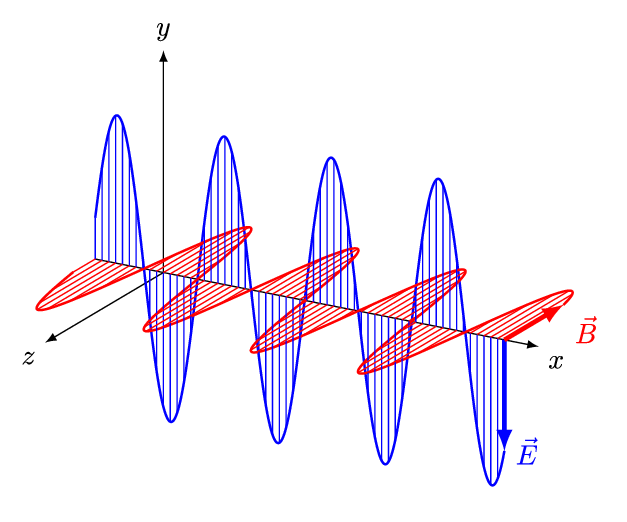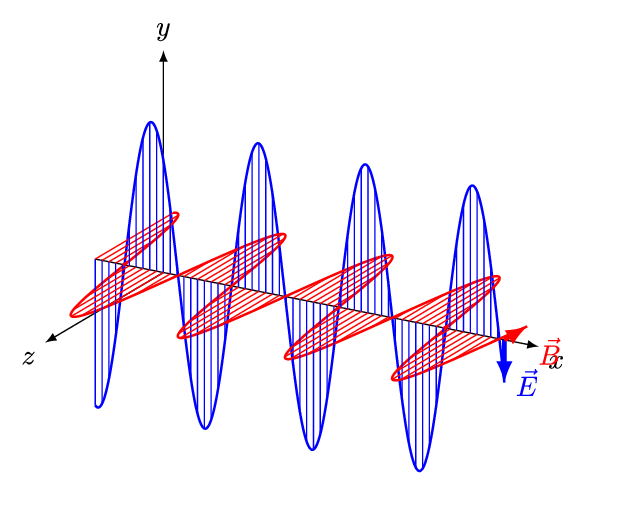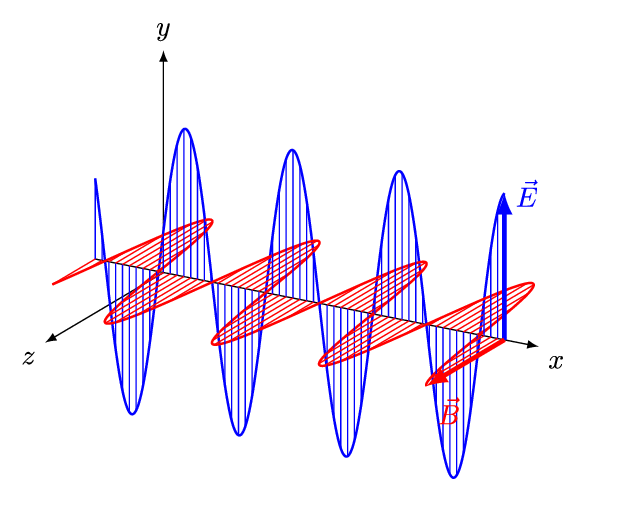
If we superpose two oppositely handed helical waves (by placing one on top of the other) they interfere to produce a linearly polarized wave. In such a wave, the electric and magnetic parts oscillate along perpendicular lines so that the electric and magnetic parts of the wave can be more easily distinguished.
The spatial distance between wave crests is the wavelength (\(\lambda\)) of the light wave, while the temporal delay between successive crests reaching a fixed position is the period (\(T\)) of the light wave. Similarly, the spatial frequency (\(k\)) (or wave number) is the number of wavelengths that fit in a particular length, while the temporal frequency (\(\nu\)) (or just frequency) is the number of periods that fit in a particular duration (usually one second). The wavelength \(\lambda\) and frequency \(\nu\) of a wave are naturally related by its constant speed: \(c = \lambda \nu\). The different wavelengths and frequencies that light can have form an electromagnetic spectrum.
The electromagnetic spectrum contains a wide range of seemingly different phenomena, ranging from radio waves (like in your car) to microwaves (like in your kitchen) to infrared (like radiative heat from stoves) to visible light (like the entire rainbow your eyes can see) to ultraviolet light (that causes sunburns) to X-rays (like those at the hospital) to \(\gamma\)-(gamma)-rays (like the cosmic rays that hit Earth from deep space). As electromagnetic waves, their only difference is their wavelength and frequency.
1.1.2.4. Light as Photons¶
In 1900, Planck noted that light was not only an electromagnetic wave, but also must be composed of small discrete chunks that he called quanta (or the smallest quantities). Arguing from the perspective of blackbody radiation and thermodynamics, Planck postulated a universal constant \(h\) that relates the frequency \(\nu\) of a photon to its energy \(E\) as \(E = h\nu\). Rather than smoothly transitioning from bright light to darkness, light becomes lumpy when it is sufficiently dim. Planck won the Nobel prize for this discovery in 1918. The quanta of light later became known as photons.
These photons were initially detected because each absorbed packet of light scatters electrons off a metal detector in proportion to the frequency and not the brightness of the light, which creates curious threshold effects in observed currents. Einstein made this connection between frequency of photons and the resulting electron energy of metals in 1905, called the photoelectric effect, which later won him the Nobel prize in 1921.
Importantly, the connection between frequency and photon energy means that the electromagnetic frequency spectrum can be read equivalently as an energy spectrum for the photons composing the electromagnetic waves. Different forms of electromagnetic radiation arise from distinct photon energies, with high energy photons corresponding to high frequency (and short wavelength) waves and low energy photons corresponding to low frequency (and long wavelength) waves. The higher the photon energy, the harder it is to create many of them, which is why higher-energy X-rays and gamma-rays tend to behave more like individual photons rather than waves containing billions of them.
Note
Electronic detectors have now become sensitive enough to see individual photons of light being absorbed in real time. The following image from 2014 is an oscilloscope trace from a superconducting nanowire acting as a photon counter. The clearly separated spikes correspond to individual photon absorptions being counted.
Note
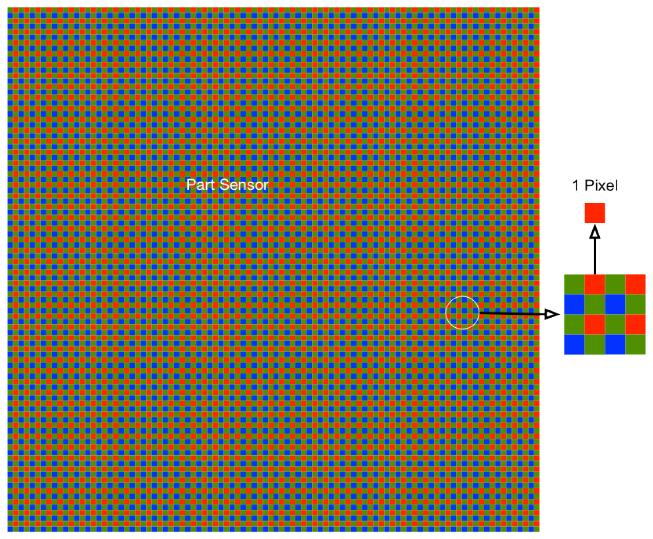
Digital cameras, such as in your smartphone, are now so sensitive that they must understand light at the level of individual photons to get a clean image. That is, the camera sensor is divided up into a grid of pixels that can each absorb individual photons. Each pixel has a red, green, or blue filter on it, so it only allows one color (frequency) of photon to be absorbed. When an image is taken, the sensor collects individual photons for a short duration of time, then records the discrete chunks of voltage produced by the integer number of photons absorbed by each pixel in the array. Later on, this pixel count data is processed and weighted by the red/green/blue filter information to reconstruct larger “color pixels” that form the entire image.
1.1.2.5. Subatomic Structure¶
By the early 1900’s it also became clear that there must also be substructure to each atom in the periodic table. In 1897 Thomson provided conclusive evidence of isolated and negatively charged electrons that were smaller than the elements of the Periodic Table, and thus subatomic in structure. This implied that atoms could be decomposed into electrons and complementary positively charged nuclei, but it was initially unclear what form such nuclei would take.
In 1911 Rutherford determined nuclear structure by scattering radiated alpha particles off gold foil. If the positive charge was evenly spread throughout the atom (as people initially expected), then the scatter would be weak. However, Rutherford found that a small number of particles scattered straight back as if they hit a dense wall. This finding implied that the majority of each atom must be empty space, with a tiny dense core of positive charge. The electrons inhabited a diffuse cloud surrounding this tiny dense nuclear core, as in the Helium atom illustrated below.
The theoretical framework of quantum mechanics was developed by physicists over the next decade to explain how this subatomic structure could possibly exist as a stable configuration. The development led to an explanation of electron orbitals (or shells) that corresponded precisely to the block structure in the Periodic Table of the elements. The subjects of physics and chemistry had merged into physical chemistry (or chemical physics) since quantum mechanics now provided a physical explanation for the observed chemical structure and phenomena.
Each chemical shell corresponds to a spatial configuration of how the electrons arrange themselves around the nucleus. As an example, the plots below visualize electron shells (n,l,m) for a Hydrogen atom, where n=0 is the “s”-shell, n=1 are the 3 “p”-shells, n=2 are the 5 “d”-shells, n=3 are the 7 “f”-shells, and so on. Each shell fits two electrons such that they have opposite directions of a new property called spin, which produces the observed block structure in the Periodic Table.
The negative electron charges and positive nucleus charges balance out to zero for each stable atom, like the Hydrogen atom centered below. However, electrons can be stripped away from or added to the nuclei, created charged ions. Positively charged ions are called cations while negatively charged ions are called anions.

Diffuse collections of ions and free electrons form the state of matter known as a plasma, which we observe in flames, fluorescent lights, stars, or near Tesla coils like the animations below.
1.1.2.6. Nuclear Structure¶
Nuclei further decompose into clusters of positively charged particles called protons (found in 1917) and uncharged particles called neutrons (found in 1920). The various atomic isotopes were found to have the same number of protons and electrons (and thus have identical chemistry), but differing numbers of neutrons.
Most isotopes are unstable and decay back to stable configurations by emitting radiation. The image below shows the three types of nuclear radiation: alpha (emitting a helium nucleus), beta (emitting an electron), and gamma (emitting a photon).

1.1.2.7. Antimatter¶
In 1932 electrons were found to have an “antiparticle” known as a positron, that had all the same properties but reversed such that when it collided with an electron they annihilated into light (photons). The development of the theoretical framework of relativistic quantum mechanics by Dirac predicted the existence of the positron, which was later confirmed experimentally. Later it was found that all matter particles have corresponding antiparticle partners.
1.1.2.8. Weak Interaction¶
In 1933 a new type of interaction called the weak interaction was postulated to account for the decay of isotopes that produced radiation. To account for the creation and annihilation of particles that occur during radiation decay, the theoretical framework of relativistic quantum field theory was developed, which is still the most successful physics framework. The Standard Model that accounts for all fundamentals we have been able to observe is expressed in the framework of relativistic quantum field theory.
1.1.2.9. Neutrinos¶
In 1934 a new particle called a neutrino was postulated due to a small amount of missing energy after a radiative decay. This particle was detected in 1943.
1.1.2.10. Muons¶
In 1936 an unexpected new particle called a muon was detected that looked identical to an electron but was much heavier. It was also unstable and decayed into more stable particles. Its identity was not fully understood until the late 1960s, after which an associated muon-neutrino was postulated (and finally detected in 1968).
1.1.2.11. Hadrons, Baryons, Mesons, Quarks, and Subnuclear Structure¶
Investigations of radiation, isotopes, and the results of smashing particles together in energetic particle accelerators from the 1930’s onward revealed many new types of particles called hadrons that were also not part of the periodic table. So many hadrons were discovered in fact that the collection of hundreds of new particles became known as a “particle zoo” that baffled people for decades.
In 1964 it finally became clear that protons, neutrons, and all the hadrons could themselves be decomposed into even smaller pieces known as quarks. The image below shows the dramatic differences in size scales for the various “sub-atomic” constituents of an atom.

Remarkably, all particles in the zoo were found to be various combinations of only 6 basic quarks (and their antiquarks) composed of three similar pairs: (up, down), (charm, strange), (top, bottom). The last of the quarks were confirmed experimentally by 1977. Interestingly, the quarks had fractional electric charge (+2/3 electron charge for up, or -1/3 electron charge for down) and also had a new type of 3-valued color-charge (with values “red”, “green”, “blue” such that the sum of all three was a neutral “white”). The hadrons known as mesons were found to be quark-antiquark pairs, while the hadrons known as baryons were found to be collections of three quarks with a net neutral color charge. Importantly, the most common baryons were the proton (composed of up, up, down) and the neutron (composed of up, down, down). The quarks were held together by exchanging new particles called gluons to produce a new interaction called the strong interaction. The image below shows the baryons of the proton and neutron, as well as the longer-range nuclear binding mesons pi+ and pi-, to illustrate how the quarks combine and how the spins and charges combine to yield the composite particles.

1.1.2.12. Higgs Mechanism¶
In 1964 the BEHGHK/Higgs particle was also postulated, as part of a mechanism by which all particles obtained their rest mass-energy. This particle was finally observed in 2012.
1.1.2.13. Electroweak Unification¶
In 1968 it was realized that the weak interaction and the electromagnetic interaction actually unified at high energy to become the same interaction: the electroweak interaction. After detailed calculations it was realized that in addition to the photon for the electromagnetic force, there should be two additional force-carrying particles (Z and W) that mediated the weak interaction. While Z is charge-neutral, W can either have positive or negative electric charge. These additional particles were observed in 1983.
1.1.2.14. Tauons¶
In 1971 a third electron-like particle called the tauon was postulated, which was even heavier than the muon, along with an associated tau-neutrino. In 1974 both the tauon and the tau-neutrino was detected and confirmed.
1.1.3. The Standard Model Revisited¶
With the discovery of the Higgs boson in 2012, all observed matter and interactions are accounted for, except for gravity. A “graviton” is expected to mediate the gravitational interaction just like the other interactions, but it may be too difficult to isolate and observe on its own. The resulting set of fundamental particles after this long journey is the Standard Model of Particle Physics (see this neat interactive table).
1.1.3.1. Quanta¶
We call each fundamental piece of the Standard Model a distinct type of quantum, meaning “one unit of a quantity”, such that many units are called quanta. This name reflects the fact that we can count integer amounts of each distinct type of quanta that compose everything in the world we see around us.
Each quantum has only two fundamental properties that distinguish it from other quanta:
rest mass-energy : affects linear motion
spin angular momentum : affects rotational motion
It is no accident that these properties are directly related to the possible motions that can occur. We will explore this connection in later sections.
Curiously, spin only occurs in integer or half-integer multiples of a fundamental unit \(\hbar\). Quanta with half-integer spin (e.g., \(\hbar/2\) or “spin-1/2”) are called fermions and naturally repel each other to maintain distinct locations and identities. Quanta with integer spin (e.g., \(\hbar\) or “spin-1”) are called bosons and naturally bunch together into collections that behave in unison. The matter or material substance around us tends to be composed of fermions, while the interactions affecting that matter are mediated by bunches of bosons.
In total, the Standard Model contains only \(2\times(3\times4) + 6 = \mathbf{30}\) distinct types of quanta. The basic quanta include 1 boson that confines rest mass-energy, 2 bosons that mediate long-range interactions, 3 bosons that mediate short-range interactions within atomic nuclei, and 4 fermions that compose stable matter. There are additionally 2 unstable “generations” (or slightly heavier copies) of the 4 matter fermions, and \(3\times4 = \mathbf{12}\) fermions of associated anti-matter (or copies of matter with inverted properties). Omitting anti-matter for brevity, the quanta are:
Interaction (bosons) : mediate changes in substance
Inertia (1): long-range, confines rest mass-energy
BEHGHK/Higgs (\(H\))
Gravity (1): long-range, only attractive, (not yet observed)
Graviton (\(G\))
Electromagnetic (1): long-range, attractive and repulsive
Photon (\(\gamma\))
Weak (2): short-range, nuclear decay/radiation
\(W\), \(Z\)
Strong (1): short-range, nuclear binding
Gluon (\(g\))
Matter (fermions) : form persistent substance
Leptons (2\(\times\)3): compose chemical shells
Electron (\(e\)), Muon (\(\mu\)), Taon (\(\tau\))
Electron-neutrino (\(\nu_e\)), Muon-neutrino (\(\nu_\mu\)), Taon-neutrino (\(\nu_\tau\))
Quarks (2\(\times\)3): compose atomic nuclei
Up (\(u\)), Charm (\(c\)), Top (\(t\))
Down (\(d\)), Strange (\(s\)), Bottom (\(b\))
The chart below neatly vizualizes the 18 basic quanta and their distinctions (omitting the 12 anti-matter quanta):
Note that only the first generation of matter (electron, electron-neutrino, up quark, down quark) are stable and can persist for long times without decaying/transforming into other quanta. Moreover, the short-range interactions stay confined within the tiny nuclei of atoms, while the presence of the Higgs interaction is observed only indirectly within the rest mass-energy of the other quanta. Therefore, the vast majority of our daily experience involves only three stable matter quanta (electron, up quark, down quark) and the two long-range interaction quanta (photon, graviton).
1.1.3.2. Interactions / Fundamental Forces¶
Each type of quantum can also have a few more properties that determine how it interacts with other quanta:
energy-momentum : affects motion, inertia, and gravitational interaction
electric charge : affects electromagnetic and weak interactions
color charge : affects strong interactions
chirality/handedness : affects weak interactions